A. A. Arzamastsev1, F. Bea2, L. V. Arzamastseva1, and P. Montero2
1Geological Institute, Kola Science Center, Russian Academy of Sciences,
Apatity
2University of Granada, Dept. of Mineralogy and Petrology, Fuentenueva
s/n., 18002,
Granada, Spain
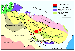
In the northeastern Baltic Shield, the Paleozoic stage of tectonic and magmatic reactivation involved generation of alkaline plutonic complexes, which have been customarily divided into two rock suites: (i) alkaline ultramafic rocks associated with carbonatites (Kovdor, Vuoriyarvi, Afrikanda, Seblyavr, etc., massifs) and (ii) agpaitic nepheline syenites, represented in the vast Khibiny and Lovozero plutons. Available isotope ages indicate that all the Paleozoic alkaline massifs of the Kola province were coeval, and their parental melts were derived from the same mantle sources [Kramm and Kogarko, 1994; Kramm et al., 1993]. The hallmark of the region's alkaline rocks is their immense abundances of REE, Y, Sr, Zr, Hf, Nb, Ta, and Th. These are either concentrated in apatite, titanite, perovskite, and other accessories or, given their concentrations in melts were high enough, form their own discrete minerals, such as loparite, pyrochlore, and eudialyte, whose economic deposits provide the basis for the region's mining industry.
Geologic observations and experimental evidence suggest that alkaline ultramafic rocks forming parts of carbonatite complexes originated through crystal fractionation in nephelinitic melts, which resulted, at early stages, in olivine, clinopyroxene, and melilite cumulates and their complementary foidolites and nepheline syenites [Arzamastsev et al., 2001; Dawson et al., 1995; Ivanikov et al., 1998; Kukharenko et al., 1965; Nielsen, 1994; Verhulst et al., 2000]. Available data on behavior of incompatible elements during magmagenesis suggest that with advancing magma crystallization, elements such as Sr, Zr, Hf, Nb, Ta, Th, and REE are likely to become enriched in terminal melt derivatives of the alkaline ultramafic series. This is indeed the case in alkaline ultramafics of the Khibiny massif, at whose lower horizons large fragments of such bodies are found surrounded by agpaitic syenites [Arzamastsev et al., 1998; Galakhov, 1975]. However, in the majority of alkaline ultramafic plutons region-wide, terminal crystallization products (ijolites, nepheline syenites, and cancrinite syenites) are appreciably depleted in elements such as Nb, Ta, and rare earths.
This work is intended to study how the above trace elements behave in alkaline ultramafic suites and, in particular, to find out why rare earth elements follow different distribution patterns in ultramafic carbonatite intrusions and in the Khibiny massif. Our study draws on mineralogic and geochemical data on representative samples from the Kovdor, Vuoriyarvi, Cape Turiy, Salmagora, and Afrikanda massifs, the Lesnaya Varaka and Ozernaya Varaka intrusions, and the Khibiny complex. ICP-MS analyses were made on whole-rock samples and on separates of coexisting perovskite, apatite, titanite, clinopyroxene, melilite, olivine, nepheline, and magnetite. Data obtained enable us to identify those factors responsible for the diversity of REE distribution patterns in alkaline ultramafic suites and, in particular, to assess the role of high-REE accessory phases-loparite, apatite, and titanite.

|
| Figure 1 |
|
| Figure 2 |

|
| Figure 3 |
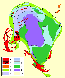
As regards the Lovozero massif, alkaline ultramafics are encountered mostly in its northeast part, where large blocks and xenoliths of peridotites and melilitic rocks occurring among nepheline syenites suggest that these rocks originated at the earliest stage of emplacement of the complex. Foidolites are not widespread in the massif, their presence being evidenced by sporadic, Ne-syenite-hosted, ijolite and melteigite xenoliths, recovered in cores from a number of drillholes.
Sixty-nine representative samples, collected from drillcores and outcrops in the Khibiny, Lovozero, Kovdor, Afrikanda, Cape Turiy, Lesnaya Varaka, Ozernaya Varaka, Seblyavr, Salmagora, Sallanlatva, Vuoriyarvi, and Ivanovka intrusions, were selected for this study from a total of more than 500 samples. Modal-mineral compositions of the samples are listed in Table 1.
Whole-rock major element analyses were carried out at the Geological Institute, Kola Science Center, Russian Academy of Sciences, using a routine technique of sample fusion with 2Na2CO3 + 1Na-tethraborate followed by dissolution in HCl. Si, Al, Mg, Ca, Fe, Ti, Ni, Co, Cr, and V were measured by atomic absorption on a Perkin Elmer 403 instrument with an accuracy (coefficient of variation, CV) better than 3% (G. Gulyuta, analyst). Na, K, Li, Rb, and Cs were measured by flame photometry (CV ~5%); P and S, by photocolorimetry and polarography from the same solutions with an accuracy of ~5% and ~10%, respectively; and F and Cl, by the ion-selective electrodes method (CV ~15%). The Fe2+/Fe3+ ratio was measured by titration (CV ~10%). H2O and CO2 were analyzed by the gravimetric method (CV ~10%).
Trace elements were analyzed by ICP-MS at the University of Granada. Sample charges
of
0.1 g were allowed to stand for 30 min in an HNO3
+ HF mixture in Teflon-lined containers
at T = 180oC and ~200 p.s.i., evaporated until dry, and dissolved in
100 ml of 4% HNO3.
Each specimen was analyzed three times on an ELAN-5000 PE SCIEX instrument using
a
rhenium within-lab standard. Accuracies were
 2 rel.% and
2 rel.% and
 5 rel.% or better for
concentrations of 50 and 5 ppm, respectively.
5 rel.% or better for
concentrations of 50 and 5 ppm, respectively.
Major elements in minerals were measured at the Geological Institute, Kola Science Center, on a Cameca MS-46 ion microprobe using natural and synthetic standards. Acceleration voltage was 30 kV for Sr and Zr and 20 kV for the rest of the elements; sample current was 20-40 nA, and ion beam diameter, 1.5-3 m m.
Mineral grains separated for trace-element analysis were inspected under an optical microscope in order to reject foreign inclusions. A number of samples were inspected under a scanning electron microscope. Minerals were separated on a magnetic separator and in heavy liquids. Finally, 8-10 mg charges were cleaned by repeated hand-picking to 99.9 vol % purity. Trace element abundances in sample charges were measured by ICP-MS as described above.
|
| Figure 4 |
Ultramafic rocks encountered in the Khibiny and Lovozero massifs are represented by peridotites and, chiefly, by pyroxenites, which are texturally and compositionally similar to ultramafics of the Kovdor suite. Pyroxene with characteristic cumulus features is in places partially replaced by richterite and/or phlogopite. REE-bearing accessories are represented by apatite and titanite (up to 6 and 2 vol %, respectively; Table 1). An important distinction is that perovskite is a rare accessory in all ultramafic rocks from the Khibiny.
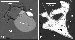
Melilite-bearing rocks, dominantly uncompahgrites and turjaites, form independent intrusion phases in the Kovdor and other alkaline ultramafic massifs. In the Khibiny and Lovozero, melilite rocks are encountered as xenoliths. Melilite together with phlogopite compose large oikocrysts with clinopyroxene and nepheline inclusions. REE-bearing phases, represented by perovskite, apatite, and, less frequently, titanite, occur sporadically. Intersticial segregations of magnetite are not infrequently rimmed by secondary perovskite (Figure 4b), indicative of a Ti-bearing phase having been exsolved from primary titanomagnetite [Nielsen et al., 1997].
Foidolites occur widely in both the Kovdor and Khibiny alkaline ultramafic suites. The rocks are ortho- and mesocumulates with nepheline segregations varying in habit from anhedral to euhedral in less and more leucocratic rocks, respectively. Clinopyroxene makes up zoned segregations whose compositions are different in the Kovdor and Khibiny suites. In foidolites of the Kovdor and other alkaline ultramafic massifs, pyroxene is represented by diopside, which makes up grain cores, while grain rims are composed of aegirine-augite. Pyroxenes in Khibiny ijolite-melteigites are more alkaline than in the Kovdor suite and, unlike the latter, are composed of aegirine-augite rimmed by aegirine. Accordingly, late amphiboles developed after clinopyroxene have different compositions, mostly pargasitic in Kovdor foidolites and corresponding to richterite or magnesiocataphorite in the Khibiny suite.
Distribution patterns of REE-bearing accessories are different in Kovdor and Khibiny foidolites. In Kovdor, Vuoriyarvi, and Cape Turiy melteigites and ijolites, the early-generation perovskite, just as apatite and titanite, is a typical accessory phase. Not infrequently, the perovskite is observed to be replaced by titanite. The mean titanite abundance in ijolites is < 1 vol %. Apatite abundances in foidolites attain a critical maximum of 1.2 wt % in the most melanocratic lithologies [Arzamastseva and Arzamastsev, 1996].
Unlike the Kovdor suite, Khibiny foidolites contain apatite and titanite not only as late magmatic accessories, but also as widespread primary REE minerals. Both apatite and titanite are most abundant in melanocratic foidolite varieties, where they account for 4 and 5 vol % on average, respectively. A distinctive feature of Khibiny foidolites is that they lack primary perovskite.
Nepheline- and cancrinite syenites in Kovdor-type massifs show evidence of early crystallization of light-colored phases, nepheline and K-Na-feldspar. Just as in foidolites, pyroxene is represented by zoned grains of diopside rimmed by aegirine-augite. REE-bearing accessories are titanite and apatite.
Arzamastsev et al. [1998] showed that neither Khibiny nor Lovozero agpaitic Ne-syenites are cogenetic with rocks of the alkaline ultramafic series. This is evidenced, in particular, by the fact that the REE-bearing mineral assemblage in agpaitic syenites differs fundamentally from that in the alkaline ultramafic rocks. Lovozero lujavrites have as much as 90 vol % eudialyte and 12 vol % loparite, while in Khibiny Ne-syenites the most widespread primary magmatic phases are eudialyte and apatite. Distribution, composition, and origin of these economically important minerals were studied by Kogarko [1977, 1999] and Kravchenko et al. [1992] and are beyond the scope of this work.

|
| Figure 5 |
The following distinctions exist between the Kovdor and Khibiny suites in terms of major-element abundances. Khibiny foidolites are relatively lower in MgO and higher in SiO2 and alkalis (Figure 5). Thus, weighted mean abundances of these oxides in Khibiny foidolites are 43.62 wt % SiO2, 9.40 wt % Na2O, 3.59 wt % K2O, and in Kovdor-type foidolite intrusions, 41.64 wt % SiO2, 8.10 wt % Na2O, 2.73 wt % K2O. The high contents of silica and alkalis are manifest in modal-mineral compositions of Khibiny foidolites, such that K-Na-feldspar is a characteristic accessory mineral in ijolites, where it accounts for as much as 10 vol %. Among other distinctions between Kovdor and Khibiny rocks, one should note the higher F content of Khibiny rocks and the differences in TiO2 and P2O5 distributions. Thus, in Kovdor suite the highest TiO2 abundances are detected in pyroxenites, which have 8-15 wt % MgO on average, whereas in Khibiny suite TiO2 is highest in the most evolved rocks, ijolites and melteigites, which have 3-7 wt % MgO.
|
| Figure 6 |
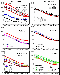
All the Kovdor-type alkaline ultramafic rocks lack Eu anomaly and show depletion
in the
light REE relative to the heavy REE (Figure 6). The lowest total REE contents and (La/Yb)N
ratios (11.7-17.4) were detected in olivine cumulates from the Kovdor,
Lesnaya Varaka, and
Salmagora massifs. By contrast, pyroxenites from the majority of massifs, which contain
perovskite and, to a lesser extent, apatite, are sharply REE-enriched relative to
the mean
composition of alkaline ultramafic rocks. These rocks have steeper REE patterns with
(La/Yb)N = 52-226. More evolved derivatives (melilitolites, foidolites, and
Ne/cancrinite
syenites) have lower REE contents compared to the mean alkaline ultramafic rock
composition for the province, at 5
 10 to 3
10 to 3
 10
2 times the chondritic level. Therefore, with
advancing differentiation of the Kovdor suite, late-stage ijolite and Ne-syenite
derivatives
become progressively depleted in REE.
10
2 times the chondritic level. Therefore, with
advancing differentiation of the Kovdor suite, late-stage ijolite and Ne-syenite
derivatives
become progressively depleted in REE.
Khibiny alkaline ultramafic rocks have a REE distribution
pattern which is sharply
dissimilar to the Kovdor suite (Figure 6). By and large, REE abundances of perovskite-free
peridotites and pyroxenites are close to the estimated mean values for the alkaline
ultramafic
series. Due to low (La/Yb)N ratios, ranging 31.4-53.4, the light REE contents are 0.5-0.7
times the mean values for the alkaline ultramafic series, whereas the medium and
heavy REE
contents are 2 times higher than the mean values. Just as in pyroxenites, REE abundances
in
Khibiny melilite rocks are within the range of mean values established for the alkaline
ultramafic rocks. On the other hand, the latest derivatives in the suite, ijolites
and
melteigites, are markedly REE-enriched relative to the mean composition of alkaline
ultramafic rocks of the province. In particular, melteigites from the Khibiny layered
complex, which have up to 8 vol % titanite and 5 vol % apatite,
show 2
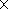 103 times the
chondritic REE concentrations. To sum up, the Khibiny alkaline ultramafic suite is
characterized by a progressive REE enrichment of its late derivatives.
103 times the
chondritic REE concentrations. To sum up, the Khibiny alkaline ultramafic suite is
characterized by a progressive REE enrichment of its late derivatives.

|
| Figure 7 |
Apatite. Besides Ca, P, and F, apatites from all the rocks under study have appreciable abundances of Sr, Y, and REE (Table 6), the late apatite generation having highest values. Apatites from pyroxenites and foidolites of the Kovdor, Afrikanda, Sallanlatva, and Khibiny plutons have very narrow ranges of both total REE contents and (La/Yb)N, 57-278. Chondrite-normalized REE spectra (Figure 7) for apatites are nearly parallel and broadly correlative to the REE abundances of host rocks. The earlier report on Eu anomaly [Kravchenko et al., 1979] has not been confirmed by any single measurement. Comparison with other provinces in terms of REE contents and patterns shows that apatite compositions under study resemble those from carbonatites of the Alno, Sokli, and Fen massifs [Hornig-Kjarsgaard, 1998].
Titanite. We have analyzed late titanites, which replace perovskite in Afrikanda pyroxenites (sample 25-AFR), and groundmass titanites from Ozernaya Varaka and Khibiny ijolites. Comparison shows that Khibiny varieties are appreciably enriched in Sr, whereas Ozernaya Varaka and Afrikanda titanites have elevated concentrations of Zr, Hf, U, and Th (Table 7). All the varieties have narrow ranges of REE contents and relatively low (La/Yb)N ratios (40-53), which results in gently sloping, straight chondrite-normalized patterns (Figure 7).

|
| Figure 8 |
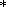 <0.08) was
established in Lesnaya Varaka dunites, which contain significant amounts of magnetite.
Based on this evidence, we assume that a considerable fraction of REE contained in
olivine
grains is concentrated not in this mineral proper, but in perovskite and magnetite
microinclusions.
<0.08) was
established in Lesnaya Varaka dunites, which contain significant amounts of magnetite.
Based on this evidence, we assume that a considerable fraction of REE contained in
olivine
grains is concentrated not in this mineral proper, but in perovskite and magnetite
microinclusions.
Clinopyroxene. In early cumulates of the alkaline ultramafic series, clinopyroxene is represented by diopside Di80Hd15Ac5, with foidolites and nepheline syenites containing zoned segregations that range in composition from augite Di55Hd40Ac5 to aegirine-augite Di50Hd30Ac20 [Arzamastsev and Arzamastseva, 1993; Kukharenko et al., 1965]. A study of zoned pyroxene grains using the laser ablation technique [Arzamastsev et al., 2001b] shows that zone-to-zone variations of major elements within crystals are not accompanied by any marked variations in the contents of trace elements, in particular, REE. This is further supported by analyses of pyroxene separates from pyroxenites, melilitolites, and ijolites from carbonatite massifs of the province, which reveal a narrow range of REE variations in all the groups of rocks (Table 8). The higher REE contents are observed in rock varieties from Khibiny ijolites, although all the rocks have the same REE distribution pattern. Plots shown in Figure 8 demonstrate all the clinopyroxenes to be enriched in Yb and Lu relative to Dy, Ho, and Er.
Melilite. Microprobe measurements on melilites from rocks of the Kovdor massif
yield the
following composition (mol %): Mg-akermanite, 49-70; Fe-akermanite, 5-11; Na-melilite,
36-40. Compared with previously published data on melilite compositions in the rocks
of the province
[Bell et al., 1996],
the analyzed melilites have higher Mg/Fe ratios, a feature typical
of Ne-free alkaline ultramafites
[Mitchell, 1996a;
Rass, 1986].
Overall, the melilite is high
in Sr due to the isomorphous replacement Sr2+ Ca2+. Unlike Khibiny melilites, Kovdor
ones display no appreciable enrichment in Sr (Table 8). Compared
with the scanty reported
REE measurements on melilites from various provinces
[Mitchell, 2001;
Onuma et al., 1981],
Kovdor specimens have somewhat lowered total REE contents and straight patterns
with relatively high (La/Yb)N ratios of 178-203
(Figure 8).
Ca2+. Unlike Khibiny melilites, Kovdor
ones display no appreciable enrichment in Sr (Table 8). Compared
with the scanty reported
REE measurements on melilites from various provinces
[Mitchell, 2001;
Onuma et al., 1981],
Kovdor specimens have somewhat lowered total REE contents and straight patterns
with relatively high (La/Yb)N ratios of 178-203
(Figure 8).
Nepheline. Nepheline compositions in rocks of the Kovdor-type alkaline ultramafic
intrusions range from Ne77.8-82.5Ks9.6-18.9Qz1.4-3.2 to
Ne78.6-81.6Ks15.2-20.1Qz1.4-3.2 in foidolites
and Ne-syenites, respectively. Nephelines
from Khibiny foidolites are higher in the kalsilite end-member
and silica (Ne68.4-72.8Ks21.5-26.1Qz4.8-7.4
). Because of high Fe2O3 contents in the matrix
of nepheline, all the nepheline grains are replete with aegirine microlites representing
exsolution products. REE contents in all the nepheline varieties range from low to
very low
(Table 8).
Chondrite-normalized REE patterns are straight (Figure 8), nephelines from Khibiny
ijolites having negative Eu anomaly (Eu/Eu
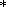 = 0.13), evidently due to the presence
of
magnetite microinclusions, rather than aegirine ones only. On the other hand, in
nephelines
from Kovdor ijolites, which are least abundant in aegirine microlites, Eu anomaly
is
expressed poorly (Eu/Eu
= 0.13), evidently due to the presence
of
magnetite microinclusions, rather than aegirine ones only. On the other hand, in
nephelines
from Kovdor ijolites, which are least abundant in aegirine microlites, Eu anomaly
is
expressed poorly (Eu/Eu
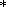 = 0.69).
= 0.69).
Data on REE distribution in principal REE- bearing phases--perovskite, apatite, and titanite (Tables 6, 7)--enable us to calculate partition coefficients for these phases. Comparison of coefficients obtained for the coexisting pairs perovskite/apatite (DPrv/Ap ), perovskite/titanite (DPrv/Tit ), and apatite/titanite (DAp/Tit ) (Table 9) shows that in early pyroxene-perovskite cumulates, REE partition preferentially into the perovskite. Overall, during magmatic crystallization, REE enter the above minerals in the following order: perovskite > apatite > titanite. According to our calculations, the early-generation perovskite (sample AFR-5) extracts medium- and heavy REE most strongly (DPrv/Ap for Tb-Lu > 3), whereas in perovskite IIsapatite pairs (sample 8-OV) the bulk of the heavy REE partition into the apatite. The results obtained are consistent with microprobe measurements on minerals from Ugandan clinopyroxenites and kamafugite lavas [Lloyd et al., 1996] and from the plutonic Oldoinyo Lengai alkaline ultramafic rocks [Dawson et al., 1994, 1995], which reveal a relative constancy of REE distribution in the perovskite-apatite pair (D Prv/Ap: La 9, Ce 16, Nd 9.5). DPrv/Ap values similar to those obtained by us for the late perovskite II-apatite pairs were established for Kaiserstuhl calcite carbonatites [Hornig-Kjarsgaard, 1998], DPrv/Ap in these rocks decreasing from 6.8-4.9 for the LREE to 3.0-1.5 for the MREE through to 0.5 for Yb.

|
| Figure 9 |
In calculations of D REE for minerals from the more evolved members of the alkaline ultramafic series (ijolites), we assumed their constituent phases (clinopyroxene, melilite, apatite, and titanite) to have been in equilibrium with the host rocks. The obtained DCpx/rock and DAp/rock values, listed in Table 11, broadly compare to those for minerals in alkaline volcanites from other regions [Caroff et al., 1993; Foley et al., 1996; Irving and Price, 1981; Larsen, 1979; Onuma et al., 1981]. On the other hand, partition coefficients for titanites from Khibiny and Ozernaya Varaka ijolites are lower than those for phonolite-hosted titanites [Worner et al., 1983].
Petrologic evidence, supported by experimental data [Edgar, 1987; Le Bas, 1987; Onuma and Yamamoto, 1976; Pan and Longhi, 1989, 1990; Veksler et al., 1998a; Wilkinson and Stolz, 1983], indicate that the main process responsible for the genesis of alkaline ultramafic suites of various provinces worldwide was fractional crystallization of primary olivine melanephelinitic magma. Coherence of the rock formation sequence from early olivine- and clinopyroxene cumulates to melilitolite, foidolite, and Ne-syenite, is corroborated by geologic and petrographic observations. Estimates of magma compositions for the Kola alkaline province [Arzamastsev et al., 2001a] allow the inference that alkaline ultramafics of the Kovdor and Khibiny types originated from the same primary magma. This is further supported by isotope studies, indicative of a single mantle source for Khibiny and Kovdor alkaline rocks [Kramm and Kogarko, 1994]. Variation trends in major-element plots for both suites reflect sequential precipitation of olivine, clinopyroxene, and melilite. Foidolites are produced through a reaction that involves resorption of melilite and formation of diopside and nepheline:
Published data concerning distribution of trace elements in the above principal mineral phases of the alkaline ultramafic series are indicative of REE, Sr, Y, Zr, Hf, Nb, and Ta enrichment of final derivatives. Indeed, taking into account that partition coefficients for these elements in the first phases to crystallize, olivine and diopside, and considerably lower than 1, incompatible elements should be concentrated in late ijolite and Ne-syenite melts. Such distribution is exemplified by Khibiny-type alkaline ultramafics, in which, as follows from the plot in Figure 5, late ijolites are abnormally high in REE, Sr, and Y. On the other hand, as appears from the diagram in Figure 5, within the Kovdor-type alkaline ultramafic series, late ijolites and nepheline syenites of the Maly Kovdor, Vuoriyarvi, and Ozernaya Varaka massifs are more strongly depleted in REE than early differentiates. A similar pattern is found in alkaline ultramafic suites of the Maimechas-Kotui province in Siberia [Egorov, 1991], the Gardiner complex in East Greenland [Nielsen et al., 1997], and Tanzanian plutonic alkaline suites [Dawson et al., 1995].
Let us consider the main factors responsible for the differences in REE enrichment patterns between Kovdor- and Khibiny-type alkaline ultramafic rocks, which should include (i) conditions at which the primary alkaline ultramafic magma precipitates the principal REE phases (perovskite, apatite, and titanite), and (ii) changes in the composition of the primary magma and, accordingly, in the crystallization order of principal and accessory mineral phases as a result of mixing with batches of phonolitic melt supplied from independent source.
According to experimental data [Kogarko, 1990; Veksler and Teptelev, 1990] and to studies on melt inclusions [Kogarko et al., 1991; Veksler et al., 1998b], perovskite and apatite crystallized at early stages. This is evidenced by the fact that euhedral perovskite and apatite crystals contain inclusions that were homogenized at temperatures of > 970o C and 1000-700o C, respectively [Kogarko, 1977; Nielsen et al., 1997]. Hence, crystallization paths of the alkaline ultramafic series must be considered with due account of REE-bearing phases, in the context of the six-component systems SiO2 -TiO2 -Al2O3 -CaO-MgO-Na2O and SiO2 -P2O5 -Al2O3 -CaO-MgO-Na2O.
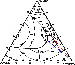
|
| Figure 10 |
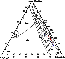
|
| Figure 11 |
According to the first scenario, which is apparently materialized
in the Kovdor-type rock
sequence, the olivine melanephelinitic melt
A of Figure 10, after having precipitated olivine,
will evolve toward the diopside-perovskite cotectic, where perovskite-clinopyroxene
cumulates are formed. Early crystallization of a phase that has D
REE 1 will result in a
dramatic depletion of residual melt, which will sequentially produce the series of
REE-depleted
derivatives (ijolites and nepheline syenites). The central role in early extraction
of
REE from melt was thus played by perovskite, although apatite may also have played
a
subordinate role at this stage, because it could, in principle, have reached the
liquidus as
well. With respect to the Kovdor-type alkaline ultramafic series, evidence for the
model just
proposed is as follows:
1 will result in a
dramatic depletion of residual melt, which will sequentially produce the series of
REE-depleted
derivatives (ijolites and nepheline syenites). The central role in early extraction
of
REE from melt was thus played by perovskite, although apatite may also have played
a
subordinate role at this stage, because it could, in principle, have reached the
liquidus as
well. With respect to the Kovdor-type alkaline ultramafic series, evidence for the
model just
proposed is as follows:
(1) The Afrikanda, Vuoriyarvi, Salmagora, and Cape Turiy massifs contain clinopyroxene-perovskite cumulates composed of primary magmatic perovskite. In some intrusions (Kovdor, Afrikanda, Ozernaya Varaka, Vuoriyarvi), local clinopyroxenite zones with up to 3% primary magmatic apatite have been found.
(2) The latest derivatives of the Kovdor series are sharply depleted in REE, Nb, Ta, and Sr relative to both the mean parental magma composition and to earlier cumulates.
(3) Primary REE-bearing accessories in late differentiates of the Kovdor series are relatively low in REE and Sr due to the melts being depleted in these elements. Thus, apatites from Ozernaya Varaka cancrinite syenites have as little as 0.67-0.71 LREE2O3 and 0.60-0.68 wt % SrO, and those from Maly Kovdor nepheline syenites, as little as 0.33-1.31 LREE2O3 and 0.59-1.07 wt % SrO [Arzamastseva and Arzamastsev, 1996].
Data on REE distribution in alkaline intrusions of various provinces indicate that early fractionation of REE-bearing phases is a characteristic feature of alkaline ultramafic suites. Thus, the Oldoinyo Lengai plutonic suite has been reported to comprise clinopyroxenite (jacupirangite) cumulates with up to 28% perovskite [Dawson et al., 1995]. The character of REE distribution in the Oldoinyo Lengai rocks corresponds exactly to that in the Kovdor series: REE are enriched in early cumulates and sharply depleted in terminal members of the series (ijolites and eucolite-bearing Ne-syenites). Another example is provided by the Maimecha-Kotui province, where the Kugda, Guli, and Odikhincha massifs are reported to contain olivine and clinopyroxene cumulates rich in primary magmatic perovskite [Egorov, 1991]. The Nizhnesayansky carbonatite massif (southern Siberia) has also been reported to incorporate a rock suite encompassing the full spectrum of alkaline ultramafites, including clinopyroxene cumulates with as much as 15% perovskite [Chernysheva et al., 1990].
The set of rock varieties that make up fragments of the alkaline ultramafic suite in the Khibiny massif, the order of their formation, and overall major-element characteristics were formerly believed to suggest similar courses of REE evolution and distribution for the Khibiny and Kovdor suites. However, according to geologic observations and geochemical data for the Khibiny alkaline ultramafic suite, generation of early olivine and clinopyroxene cumulates was not accompanied by massive precipitation of perovskite. This is the reason why REE remained in residual melt until final stages of crystallization, and REE enrichment did not occur until late ijolite derivatives started to form. Two factors preventing early crystallization of perovskite can be considered, both of them being apparently related to a drastic increase in silica activity in the melt. Firstly, the change in SiO2 activity may result from interaction of the primary olivine melanephelinitic magma with surrounding Precambrian basement rocks. This, however, is at variance with Sr and Nd isotope data, which suggest that crustal material was not involved in alkaline magmagenesis in the Kola province [Kramm and Kogarko, 1994]. Secondly, the change in SiO2 activity may have been caused by mixing of the olivine melanephelinitic magma with a more silicic melt. A likely candidate for such a melt might be the phonolitic magma that gave rise to the Khibiny agpaitic plutonic suite. This suite, according to isotope geochemical and petrologic data [Arzamastsev et al., 1998; Kramm et al., 1993], evolved independently, and its origin is related to a mantle source other than that of alkaline ultramafic melts. The main feature of agpaitic melts is their relatively higher SiO2 (52-56 wt %), alkali (Na2O + K2O > 16 wt %), and F contents. However, REE contents in agpaitic syenites, according to our data [Arzamastsev et al., 2001a], are only a little higher than in alkaline ultramafites. Hence, addition to the primary silica-undersaturated melanephelinitic magma of even minor batches of phonolitic melt would increase silica and alkali contents of the magma while changing its REE content only slightly. Indeed, in comparison to Vuoriyarvi, Ozernaya Varaka, Sallanlatva, and Kovdor ijolites, their Khibiny counterparts are much higher in SiO2, Na2O, and K2O (Tables 2, 3), and feldspar-bearing varieties are widespread among them. In the nepheline-diopside-titanite melting diagram (Figure 10), the change in the composition of crystallizing melt will be expressed in that the initial liquid of composition A shifts away from the perovskite cotectic surface A1 to produce perovskite-free olivine-diopside, diopside-melilite, and diopside-nepheline rocks (option A1-B1-C1 ). Accordingly, REE will be enriched progressively in these differentiates, and extraction of REE from melt will not occur until the melt has reached the apatite and/or titanite liquidus surface at the final stages of alkaline ultramafic petrogenesis. Therefore, evolution of the alkaline ultramafic series in the Khibiny massif was disturbed by mixing of minor portions of phonolitic melt with the primary olivine melanephelinitic magma, which led to a change in crystallization order of REE-bearing titanates and Ti-silicates and enrichment of late batches of melt in the majority of incompatible elements.
Our study of REE distribution in rocks and minerals of Paleozoic alkaline ultramafic rocks of the Kola province affords the following conclusions:
(1) REE patterns in rocks of the Kovdor, Afrikanda, Vuoriyarvi, and Salmagora massifs indicate a systematic depletion from earlier to later (ijolite and nepheline syenite) melt derivatives. Analysis of rock suites to be found in alkaline intrusions of other provinces (Maimecha-Kotui province of southern Siberia and East African province) shows that the above trend has a general character, inherent in many alkaline ultramafic suites.
(2) REE distribution in Kovdor-type alkaline ultramafic suites is controlled by crystallization of perovskite and, to a lesser extent, apatite. Primary olivine melanephelinitic melts of this series experienced crystallization of perovskite, the main REE-bearing mineral. Perovskite coprecipitating with the first phases to crystallize from melt, olivine and clinopyroxene, leads to a dramatic REE depletion of the residual melt and to formation of REE-depleted derivatives, ijolite and nepheline syenite.
(3) Petrogenesis of the alkaline ultramafic suite of the Khibiny massif was upset by mixing of minor batches of phonolitic melt with the primary olivine melanephelinitic magma, with an ensuing change in the crystallization order of REE-bearing titanates and Ti-silicates and enrichment of late batches of melt in the majority of incompatible elements. As a result, Khibiny ijolites, which are late and the most evolved derivatives of the alkaline ultramafic magma, have the highest REE concentrations, accommodated by high-REE apatite and titanite.
Anders, E., and N. Grevesse, Abundances of the elements: meteoritic and solar, Geochim. Cosmochim. Acta, 53, 197-214, 1989.
Arzamastsev, A., and L. Arzamastseva, Evolution of foidolite suites in alkaline massifs of the Kola province: Mineralogic and geochemical evidence (in Russian), Petrologiya, 1, (5), 524-535, 1993.
Arzamastsev, A., L. Arzamastseva, V. Glaznev, and A. Raevsky, Deep structure and composition of lower horizons of the Khibiny and Lovozero complexes, Kola Peninsula, Russia: A petrologic/geophysical model (in Russian), Petrologiya, 6, (5), 478-496, 1998.
Arzamastsev, A. A., A. V. Travin, and L. V. Arzamastseva, Final episode of Palaeozoic magmatism in the NE Fennoscandian Shield: 40Ar/39Ar isotope dating of diamond bearing kimberlites and picrites from the southern Kola, SVEKALAPKO, EUROPROBE project, 6th Workshop Lammi, Finland, 2001a.
Arzamastsev, A., F. Bea, V. Glaznev, L. Arzamastseva, and P. Montero, Kola alkaline province in the Paleozoic: evaluation of primary mantle magma composition and magma generation conditions, Russian Journal of Earth Sciences, 3, (1), 3-24, 2001b.
Arzamastseva, L., and A. Arzamastsev, Evolution of the Paleozoic nephelinite series of the Kola province: Evidence from P and Sr distribution, Geochemistry International, 34, 360-369, 1996.
Balagansky, V, V. Glaznev, and L. Osipenko, Early Proterozoic evolution of the northeastern Baltic Shield: A terrane analysis (in Russian), Geotektonika, 2, 16-28, 1998.
Bell, K., L. A. Dunworth, A. G. Bulakh, and V. V. Ivanikov, Alkaline rocks of the Turiy Peninsula, Russia, including type locality turjaite and turjite: a review, Canadian Mineralogist, 34, 265-280, 1996.
Carmichael, J. S. A., J. Nicholls, and A. L. Smith, Silica activity in igneous rocks, American Mineralogist, 55, 242-264, 1970.
Caroff, M., R. C. Maury, J. Leterrier, J. L. Joron, J. Cotton, and G. Guille, Trace element behavior in the alkali basalt - comenditic trachyte series from Mururoa Atoll, French Polynesia, Lithos, 30, 1-22, 1993.
Chernysheva, E., G. Nechelyustov, T. Kvitko, and B. Veis, Perovskite compositions in alkaline rocks of the Nizhnesayansky carbonatite complex (in Russian), Geokhimiya, 8, 102-108, 1991.
Dawson, J. B., J. V. Smith, and I. M. Steele, Trace element distribution between coexisting perovskite, apatite and titanite from Oldoinyo Lengai, Tanzania, Chem. Geol., 117, (1-4), 285-290, 1994.
Dawson, J. B., J. V. Smith, and I. M. Steele, Petrology and mineral chemistry of plutonic igneous xenoliths from the carbonatite volcano, Oldoinyo Lengai, Tanzania, J. Petrol., 36, (3), 797-826, 1995.
Dudkin, O., F. Minakov, M. Kravchenko, et al., Khibiny Carbonatites, 98 pp., Kola Branch of the USSR Academy of Sciences, Apatity, 1984.
Edgar, M. J., The genesis of alkaline magmas with emphasis on their source regions: inferences from experimental studies, in Alkaline Igneous Rocks, edited by J. G. Fitton and B. G. J. Upton, Geol. Soc. Spec. Publ., 30, 29-52. 1987
Egorov, L., Ijolite-Carbonatite Plutonism, 260 pp., Nedra, Leningrad, 1991.
Foley, S. F., S. E. Jackson, B. J. Fryer, J. D. Greenough, and G. A. Jenner, Trace element partition coefficients for clinopyroxene and phlogopite in an alkaline lamprophyre from Newfoundland by LAM-ICP-MS, Geochim. Cosmochim. Acta, 60, (4), 629-638, 1996.
Galakhov, A., Petrology of the Khibiny Alkaline Massif, 256 pp., Nauka, Leningrad, 1975.
Hornig-Kjarsgaard, I., Rare earth elements in sovitic carbonatites and their mineral phases, J. Petrol., 39, (11/12), 2105-2121, 1998.
Irving, A. J., and R. C. Price, Geochemistry and evolution of lherzolite-bearing phonolitic lavas from Nigeria, Australia, East Germany and New Zealand, Geochim. Cosmochim. Acta, 45, (8), 1309-1320, 1981.
Ivanikov, V. V., A. S. Rukhlov, and K. Bell, Magmatic evolution of the melilitites carbonatite-nephelinite dyke series of the Turiy Peninsula (Kandalaksha Bay, White Sea, Russia), J. Petrol., 39, (11/12), 2043-2059, 1998.
Kogarko, L., Issues of Agpaitic Magmagenesis, 294 pp., Nauka, Moscow, 1977.
Kogarko, L. N., Ore-forming potential of alkaline magmas, Lithos, 26, (1/2), 167-175, 1990.
Kogarko, L., Issues of the genesis of giant deposits of apatite and rare metals in the Kola Peninsula, Russia (in Russian), Geol. Rudn. Mestorozhd., 41, (5), 387-403, 1999.
Kogarko, L. N., D. A. Plant, C. M. Henderson, and B. A. Kjarsgaard, Na-rich carbonate inclusions in perovskite and calzirtite from the Guli intrusive Ca-carbonatite, Polar Siberia, Contrib. Mineral. Petrol., 109, (1), 124-129, 1991.
Kogarko, L. N., V. A. Kononova, M. P. Orlova, and A. Woolley, Alkaline Rocks and Carbonatites of the World, Part 2, Former USSR, 226 pp., Chapman and Hall, London, 1995.
Korobeinikov, A. N., F. P. Mitrofanov, S. Gehor, K. Laajoki, V. P. Pavlov, and V. P. Mamontov, Geology and Copper Sulfide Mineralization of the Salmagorskii Ring Igneous Complex, Kola Peninsula, NW Russia, J. Petrol., 39, (11/12), 2033-2041, 1998.
Kramm, U., and L. N. Kogarko, Nd and Sr isotope signatures of the Khibina and Lovozero agpaitic centres, Kola Alkaline Province, Russia, Lithos, 32, 225-242, 1994.
Kramm, U., L. N. Kogarko, V. A. Kononova, and H. Vartiainen, The Kola Alkaline Province of the CIS and Finland: Precise Rb-Sr ages define 380-360 age range for all magmatism, Lithos, 30, 33-44, 1993.
Kravchenko, S., A. Belyakov, P. Babushkin, and V. Kalyuzhny, Sodic nepheline syenites of the Khibiny massif: Likely derivatives of a high-Ca alkaline ultramafic magma (in Russian), Geokhimiya, 5, 685-697, 1992.
Kravchenko, S., D. Mineev, and E. Kamenev, REE and Sr in rocks and minerals of the ijolite-urtite complex of the Khibiny massif (in Russian), Geokhimiya, 7, 1035-1045, 1979.
Kukharenko, A., M. Orlova, A. Bulakh, E. Bagdasarov, O. Rimskaya-Korsakova, E. Nefedov, G. Ilyinsky, A. Sergeev, and N. Abakumova, The Caledonian Assemblage of Ultramafic and Alkaline Rocks and Carbonatites of the Kola Peninsula and Northern Karelia, 772 pp., Nedra, Moscow, 1965.
Larsen, L. M., Distribution of REE and other trace elements between phenocrysts and peralkaline undersaturated magmas, exemplified by rocks from the Gardar igneous province, South Greenland, Lithos, 12, 303-315, 1979.
Le Bas, M. J., Nephelinites and carbonatites, in Alkaline Igneous Rocks, edited by J. G. Fitton and B. G. J. Upton, Geol. Soc. Spec. Publ., 30, 53-83, 1987.
Lloyd, F. E., A. D. Edgar, and K. V. Ragnarsdottir, LREE distribution in perovskite, apatite and titanite from South West Ugandan xenoliths and kamafugite lavas, Miner. Petrol., 57, (3-4), 205-228, 1996.
Mitchell, R. H., The Melilitite clan, in Undersaturated Alkaline Rocks: Mineralogy, Petrogenesis and Economic Potential, edited by R. H. Mitchell, Mineral. Assoc. Canada, Short Course 24, 123-152, 1996a.
Mitchell, R. H., Perovskites: a revised classification scheme for an important rare earth element host in alkaline rocks, in Rare Earth Minerals: Chemistry, Origin and Ore Deposits, edited by A. P. Jones, F. Wall, and C. T. Williams, Mineral, Soc. (UK), Ser. 6, 41-76, Chapman and Hall, London, 1996b.
Mitchell, R. H., The classification of melilite clan, in Alkaline Magmatism and the Problems of Mantle Sources, pp. 117-150, Irkutsk, 2001.
Mitchell, R. H., and, S. J. B. Reed, Ion microprobe determination of rare earth elements in perovskite from kimberlites and alnoites, Mineral. Mag., 52, 331-339, 1988.
Nielsen, T. F. D., Tertiary alkaline magmatism in East Greenland: a review, in Alkaline Igneous Rocks, edited by J. G. Fitton and B. G. J. Upton, Geol. Soc. Spec. Publ., 30, 489-515, 1987.
Nielsen, T. F. D., Alkaline dike swarms of the Gardiner complex and the genesis of alkaline ultramafic complexes (in Russian), Geokhimiya, 8, 1112-1132, 1993.
Nielsen, T. F. D., I. P. Solovova, and I. V. Veksler, Parental melts of melilitolite and origin of alkaline carbonatite: evidence from crystallized melt inclusions, Gardiner complex, Contrib. Mineral. Petrol., 126, 331-344, 1997.
Onuma, K., and M. Yamamoto, Crystallization in the silica-undersaturated portion of the system diopside-nepheline-akermanite-silica and its bearing on the formation of melilitites and nephelinites, J. Fac. Sci. Hokkaido University, 4, (17), 347-355, 1976.
Onuma, N., S. Ninomiya, and H. Nagasawa, Mineral groundmass partition coefficients for nepheline, melilite, clinopyroxene and perovskite in melilite nepheline basalt, Nyiragongo, Zaire, Geochem. J., 15, (4), 221-228, 1981.
Pan, V., and J. Longhi, Low pressure liquidus relations in the system Mg2SiO4 -Ca2SiO4 -NaAlSiO4 -SiO2, Amer. J. Sci., 289, (1), 1-16, 1989.
Pan, V., and, J. Longhi, The system Mg2SiO4-Ca2SiO4-CaAl2O4-NaAlSiO4-SiO2, One atmosphere liquidus equilibria of analogs of alkaline mafic lavas, Contrib. Mineral. Petrol., 105, (5), 569-584, 1990.
Rass, I., The Paragenetic Analysis of Zoned Minerals, 144 pp., Nauka, Moscow, 1986.
Shakhmurdyan, A., and R. H. Mitchell, Evolution of perovskite chemical compositions in alkaline ultramafic rocks during metasomatic processes (in Russian), Zap. Vses. Mineral. O-va, 1, 57-69, 1998.
Ternovoi, V., Carbonatitic Massifs and Their Economic Mineralization, 168 pp., Leningrad State University, Leningrad, 1977.
Veksler, I. V., and M. P. Teptelev, Conditions for crystallization and concentration of perovskite-type minerals in alkaline magmas, Lithos, 26, (1/2), 177-189, 1990.
Veksler, I. V., Y. M. Fedorchuk, and T. F. D. Nielsen, Phase equilibria in the silica-undersaturated part of the KAlSiO4 -Mg2SiO4 -Ca2SiO4 -SiO2 -F system at 1 atm and the larnite-normative trend of melt evolution, Contrib. Mineral. Petrol., 131, (4), 347-363, 1998a.
Veksler, I. V., T. F. D. Nielsen, and S. V. Sokolov, Mineralogy of crystallized melt inclusions from Gardiner and Kovdor ultramafic alkaline complexes: Implications for carbonatite genesis, J. Petrol., 39, (11/12), 2015-2031, 1998b.
Verhulst, A., E. Balaganskaya, Y. Kirnarsky, and D. Demaiffe, Petrological and geochemical trace elements and Sr-Nd isotopes characteristics of the Paleozoic Kovdor ultramafic, alkaline and carbonatite intrusion, Kola Peninsula, NW Russia, Lithos, 51 (1-2), 1-25, 2000.
Wilkinson, J. F. G., and A. J. Stolz, Low-pressure fractionation of strongly undersaturated alkaline ultrabasic magma: the olivine-melilite-nepheline at Moiliili, Oahu, Hawaii, Contrib. Mineral Petrol., 38, 363-374, 1983.
Woolley, A. R., Alkaline Rocks and Carbonatites of the World, Part 1, North and South America, 216 pp., British Museum (Natural History), London, 1987.
Worner, G., J.-M. Beusen, N. Duchateau, R. Gijbels, and H.-U. Schminke, Trace element abundances and mineral/melt distribution coefficients in phonolites from the Laacher Sea Volcano (Germany), Contrib. Mineral. Petrol., 84, (2/3), 152-173, 1983.