 KS/
KS/ P)T,
(
P)T,
( a/
a/ T)P,
(
T)P,
( CP/
CP/ T)P,
and
(
T)P,
and
( KS/
KS/ T)P.
T)P.
We have reviewed thermodynamic properties of geomaterials necessary to study the thermodynamics of the deep interior of the Earth.
(1) In sections 2-5, it was shown that the determination of all
the second-order thermodynamic parameters requires knowledge of
values of three such parameters. In relation to EOS's, all the
thermodynamic parameters were lumped into thermal and caloric
types. A summary to finding of EOS's from experimental data was
presented. The approaches directly based on measured
thermodynamic characteristics can be formulated in the form of
partial differential equations. Of 16 third-order
thermodynamic parameters, only four (appropriately chosen) are
independent. Attention is given to the compilation of a
self-consistent database for minerals, relying on input data for
a,
KS (or
KT ),
( KS/
KS/ P)T,
(
P)T,
( a/
a/ T)P,
(
T)P,
( CP/
CP/ T)P,
and
(
T)P,
and
( KS/
KS/ T)P.
T)P.
(2) Each of the eight second-order parameters was analyzed separately, following the plan: the derivation of the identities between their P and T derivatives, the estimation of the intrinsic and extrinsic contributions to the temperature derivatives, useful simplifications of these relations and their consequences, and the explicit approximate dependences of the second-order parameters on pressure and temperature.
(3) In the analysis of thermal expansivity (section 6), the
Birch formula for
a = a(P)
at
T (or
S ) =
constant is generalized. It was shown that
a in the lower
mantle, calculated by the generalized formula, is sensitive to
assumed values of the mixed
P-T derivative of the bulk
modulus
KT, in the range of
dK 0/dT
0/dT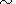 (0-4)
(0-4) 10-4
K
-1.
The assignment of a value about
2
10-4
K
-1.
The assignment of a value about
2 10-4 K
-1 for this derivative
gives
a in the lower mantle to be close to those by
the
(exponential) laws of O. Anderson et al. [1993]
and Chopelas and Boehler [1992].
Based on these estimates and our analysis, we
conclude that the coefficient of thermal expansion decreases
along the hot lower-mantle adiabat (from
P = 0 to
P = 1.35
Mbar) by a factor of 4-5. Considering the O. Anderson power law
for
a, we stated strict conditions for the consistency
of
various assumptions regarding the EOS and parameters
dT,
KT,
K
10-4 K
-1 for this derivative
gives
a in the lower mantle to be close to those by
the
(exponential) laws of O. Anderson et al. [1993]
and Chopelas and Boehler [1992].
Based on these estimates and our analysis, we
conclude that the coefficient of thermal expansion decreases
along the hot lower-mantle adiabat (from
P = 0 to
P = 1.35
Mbar) by a factor of 4-5. Considering the O. Anderson power law
for
a, we stated strict conditions for the consistency
of
various assumptions regarding the EOS and parameters
dT,
KT,
K , and
CV and cleared up the consequencies of these
assumptions. In many cases, these conditions are useful for a
self-consistent thermodynamic analysis. For example, the power
form of the Birch law,
KT
, and
CV and cleared up the consequencies of these
assumptions. In many cases, these conditions are useful for a
self-consistent thermodynamic analysis. For example, the power
form of the Birch law,
KT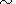 Vb
leads to
K
Vb
leads to
K = constant,
KT = KT(V),
dT = K
= constant,
KT = KT(V),
dT = K = constant,
the Murnaghan EOS (41),
and for
CV = constant,
a = a(V).
Various
extrapolations of
a to high temperatures at
P = 0 show a
great uncertainty in the resulting thermal expansivity (to
30-50% at
T 1500-2000 K), which indicates that
high-temperature measurements of
a are very neeeded to
improve the knowledge of
a.
= constant,
the Murnaghan EOS (41),
and for
CV = constant,
a = a(V).
Various
extrapolations of
a to high temperatures at
P = 0 show a
great uncertainty in the resulting thermal expansivity (to
30-50% at
T 1500-2000 K), which indicates that
high-temperature measurements of
a are very neeeded to
improve the knowledge of
a.
(4) The isobaric specific heat
CP under the lower-mantle
conditions (section 7) decreases approximately 10% along the hot
adiabat, from
P = 0 to
P = 1.35 Mbar. At low temperatures
T < Q,
the intrinsic anharmonicty competely prevails, but at
T > Q, when
( CP/
CP/ T)P is small,
its contribution is only 15-30%.
T)P is small,
its contribution is only 15-30%.
(5) The difference between the thermal pressure model of O. Anderson and the Mie-Grüneisen EOS is emphasized (section 8). This model has two specific features: in general, its thermal pressure is linear in temperature, but the volume dependence of thermal pressure depends on the kind of material. From our estimation, at T > Q, the nonlinear terms in Pth contribute no more than 1-3%. In total, we refer to the existence of, at least, four models of thermal EOS: the Mie-Grüneisen (or more general anharmonic lattice) EOS, a model with various forms of the reference isotherm P(V, T0) and with a given a(P, T) dependence, the O. Anderson model mentioned above, and the formulation of type (28) with assumed temperature variations of the EOS parameters.
(6) In section 9, the Anderson-Grüneisen
parameters
dS and
dT are analyzed in more detail.
An explicit
expression for
dT(V) at
T (or
S ) = constant was
derived from the generalized formula of Birch. We find that, for
dK 0/dT =
2.3
0/dT =
2.3 10-4 K
-1 (see the derivation of (3)),
dT at the
base of the mantle is almost half the value at
P = 0.
10-4 K
-1 (see the derivation of (3)),
dT at the
base of the mantle is almost half the value at
P = 0.
(7) The adiabatic-isothermal transformation of bulk moduli are
discussed in section 10. In addition to the previous
considerations, the useful formula (98) was derived for the
mixed derivative
dK 0/dT.
Altogether, this parameter for various
geomaterials is estimated by a value of the order of
(1-3)
0/dT.
Altogether, this parameter for various
geomaterials is estimated by a value of the order of
(1-3) 10-4 K
-1.
10-4 K
-1.
From the analyzed temperature behavior of bulk moduli, we infer
that the
dVT = K - dT
and
dVS values
at room temperature fall mostly between
-4 and
-1 and between
-1 and 1, respectively. However, their
high-temperature values are in the range from
-1 to 1 for
dVT and from
0 to 1.5 for
dVS. The
approximation
dTV
- dT
and
dVS values
at room temperature fall mostly between
-4 and
-1 and between
-1 and 1, respectively. However, their
high-temperature values are in the range from
-1 to 1 for
dVT and from
0 to 1.5 for
dVS. The
approximation
dTV 0 ( dT
0 ( dT K
K and
KT = KT(V) ) is
justified for many but not all minerals.
and
KT = KT(V) ) is
justified for many but not all minerals.
In relation to the interpretation of seismic tomography data for the lower mantle, we found the following ranges of acceptible value for this largest layer of the Earth: dTV 0.2, q 0.8, g 1.1, dT 3-3.3, and dS 1.9-2.2 (provided that the thermal interpretation of these data is true).
The KS values at high temperature, evaluated by the power law with dS = constant and by the O. Anderson enthalpy method have errors of the order of 2-6 and 1-3%, respectively. Thus, it is confirmed that the O. Anderson [1995] method is quite efficient.
(8) A number of identities for the Grüneisen
parameter
g and its logarithmic derivative
q = ( lng/
lng/ ln V)T were given in section
11.
They show that the conditions
CV = constant or
CV = CV(T) lead to
g = g(V)
or
g = f(V)/CV(T),
respectively. Both these cases are compatible to the
O. Anderson thermal pressure model, with
t = aKT
=
constant or
t = t(V).
Any of the indicated
conditions for
CV also gives
q = q(V) or
q = constant;
moreover, from the inequality
0
ln V)T were given in section
11.
They show that the conditions
CV = constant or
CV = CV(T) lead to
g = g(V)
or
g = f(V)/CV(T),
respectively. Both these cases are compatible to the
O. Anderson thermal pressure model, with
t = aKT
=
constant or
t = t(V).
Any of the indicated
conditions for
CV also gives
q = q(V) or
q = constant;
moreover, from the inequality
0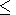 q
q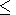 1, it
follows that
0
1, it
follows that
0 K
K - dT
- dT 1 and vice versa. Thermodynamically estimated
q values fall largely into the interval 0.5-2. This parameter
generally decreases with pressure and temperature. In the
derivative
(
1 and vice versa. Thermodynamically estimated
q values fall largely into the interval 0.5-2. This parameter
generally decreases with pressure and temperature. In the
derivative
( g/
g/ T)P,
the intrinsic anharmonicity prevails on the whole,
suggesting a significant dependence of
g on temperature.
In addition to many known expressions for
g(V), we
derived a new one based on the parameter
l
T)P,
the intrinsic anharmonicity prevails on the whole,
suggesting a significant dependence of
g on temperature.
In addition to many known expressions for
g(V), we
derived a new one based on the parameter
l 1 - (
1 - ( ln gT/
ln gT/ ln V)S 1. The
l = 0 case is reduced to the Rice [1965] formula.
Variation in
l in the interval of 0-1 (accordingly,
dVS
ln V)S 1. The
l = 0 case is reduced to the Rice [1965] formula.
Variation in
l in the interval of 0-1 (accordingly,
dVS l(1 + agT)-1 ranges approximately over the
same interval for
agT
l(1 + agT)-1 ranges approximately over the
same interval for
agT 1 ) appreciably affects the
g values at high compression.
1 ) appreciably affects the
g values at high compression.
(9) The identities and approximations for the adiabatic
temperature gradient
tS (
( T/
T/ P)S were systematized. Our
thermodynamic estimates of the Boehler parameter
n = (
P)S were systematized. Our
thermodynamic estimates of the Boehler parameter
n = ( lntS/
lntS/ ln V)T are close to his
experimental results for olivine, quartz, and
periclase. The uncertainty of the order of one in the
estimated
n is caused by errors in the used input thermodynamic
data. In the derivative
(
ln V)T are close to his
experimental results for olivine, quartz, and
periclase. The uncertainty of the order of one in the
estimated
n is caused by errors in the used input thermodynamic
data. In the derivative
( tS/
tS/ T)P,
the intrinsic anharmonic contribution
was found to dominate. When determining the EOS from data for
tS, an important role is played
by the relation of this
parameter to specific heat.
T)P,
the intrinsic anharmonic contribution
was found to dominate. When determining the EOS from data for
tS, an important role is played
by the relation of this
parameter to specific heat.
Finally, in sections 6-8 and 10, we checked on the validity of the Mie-Grüneisen EOS used to evaluate a, CP, t, and KS. Qualitatively, this EOS model correctly describes the P-T behavior of the indicated parameters, but in general, it does not always provide a sufficiently high accuracy of the estimated values. For this reason (see also the inference (8) above), it is concluded that care must be exercised when applying this type of EOS in geophysics.