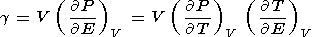
The thermodynamic Grüneisen parameter is defined by (8) or (14), which further lead to several useful identities
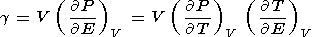 |
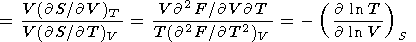 | (105) |
The typical values of g by (8) or (14) range from 1 to 2 (see, e.g., Table 2 and Pankov et al. [1997]). Of 54 minerals treated by D. Anderson [1989], only five have g greater than 2, and none has g over 3. Low values of g are seldom encountered: e.g., g = 0.4 for a -quartz, 0.3 for coesite, and even g< 0 for U 2 O, AgJ, and b -quartz.
The logarithmic derivatives of g with respect to V (or P ) are characterized by the parameter q, for which from (8) and (100), we find
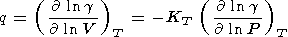 |
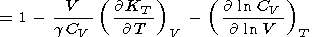 | (106) |
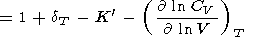 |
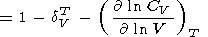 | (107) |
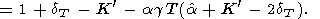 | (108) |
As noted earlier (see (57) or (69) and (8)), in general, the
CV = constant case leads to
g = g(V),
and
therefore,
q = q(V) or
q = constant. If
CV is only
temperature-dependent, there are three possibilities: (1)
q = q(V),
(2)
q = constant
1 (i.e.,
dVT = constant
0 ),
and (3)
q = 1 ( dVT = 0,
K = dT(V),
and
t = aKT
= constant). Thus, both
CV = constant
and
CV = CV(T) conditions result
in the case that the two
inequalities are equivalent:
= dT(V),
and
t = aKT
= constant). Thus, both
CV = constant
and
CV = CV(T) conditions result
in the case that the two
inequalities are equivalent:
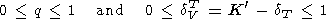 | (109) |
If we simply assume that g is only volume-dependent, then from (14), (33), and (106),
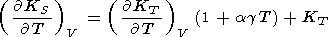 |
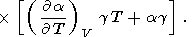 | (110) |
Placing in (110) for
(KS/ T)V
by identity (99),
T)V
by identity (99),
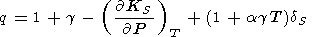 | (111) |
However, in the general case, g = g(V, T), and from the formula for g in (18), we find
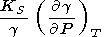 |
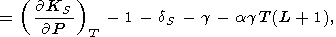 | (112) |
which, upon excluding L by (85), yields the important identity [Bassett et al., 1968]
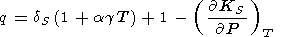 |
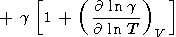 | (113) |
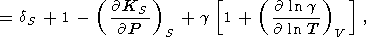 |
For g = g(V), this identity is reduced to (111).
In section 6, we have already referred to some data on values of q. In general, values of q can be inferred from the following sources: 1) thermodynamic estimation by (108) or (111), 2) fit of the Mie-Grüneisen type EOS to data on a(T), CP(T), and KS(T) at P = 0, 3) shock wave data [e.g., McQueen, 1991; Duffy and Ahrens, 1992a], 4) adiabatic temperature gradient measurements [Boehler, 1982, 1983], 5) spectroscopy of solids [e.g., Reynard et al., 1992; Williams et al., 1987], 6) theoretical EOS models [e.g., Isaak et al., 1990], 7) analysis of geophysical data [O. Anderson, 1979b; D. Anderson, 1989]. The values of q estimated by (108) and given in Table 3 and Pankov et al. [1997], fall into the interval 0.5-2, except for the high values for coesite (about 17), fayalite (2-3), and Fe-perovskite (4-5). Small negative values were also found for enstatite and FeO (probably, due to inaccurate input data). With increasing T at P = constant or with increasing P at T (or S = constant), q decreases (see also section 6).
For the temperature derivative of g, we again have the expansion of type (29)
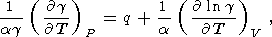 | (114) |
where the intrinsic anharmonicity term can be evaluated using (18) and (85)
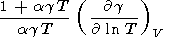 |
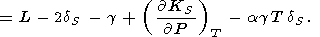 | (115) |
This term is usually negative and completely prevails in (114) at T < Q, but at high temperatures, its value is comparable to q. Thus, the frequently used assumption that g = g(V) is unsatisfactory in the general case, and the temperature effect on the Grüneisen parameter can serve as a measure of the validity of the Mie-Grüneisen EOS [Molodets, 1998].
Another suitable representation of
( g/
g/ T)V follows from (8) and (69)
[Stacey, 1977b]
T)V follows from (8) and (69)
[Stacey, 1977b]
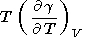 |
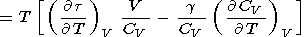 | (116) |
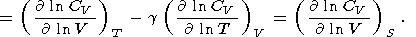 |
If g = g(V), then either CV = CV(S) or CV = constant. The case CV(S) results in
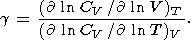 | (117) |
Moreover, (117) leads to CV(V, T) = CV(Q/T) and Q/T = f(S), so that g is represented as g = -d lnQ/d ln V, where Q is a characteristic temperature.
The frequently used volume dependences of the latice Grüneisen parameter were given in section 6. The Rice [1965] formula is also of interest
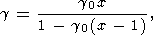 | (118) |
which is derived from (110) under the condition that
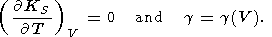 |
The inequality
( KS/
KS/ T)V > 0 (see section 10 and D. Anderson
[1989])
holds true of many materials and therefore gives a lower limit
for their dependence
g(V), i.e.,
q
T)V > 0 (see section 10 and D. Anderson
[1989])
holds true of many materials and therefore gives a lower limit
for their dependence
g(V), i.e.,
q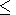 1 + g
and
g
1 + g
and
g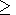 g(x)
by (118). This limit was previously
found from the Mie-Grüneisen EOS [Kalinin
and Panov, 1972],
but it also follows from the given thermodynamic consideration.
g(x)
by (118). This limit was previously
found from the Mie-Grüneisen EOS [Kalinin
and Panov, 1972],
but it also follows from the given thermodynamic consideration.
Equation (118) can be considered a partial case of the more general representation g = g(V, S). We introduce a parameter l defined as
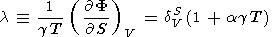 |
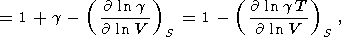 | (119) |
where F = KS/r. Assuming that l = l(S) or l = constant and using (119) and (105), we find by integration that
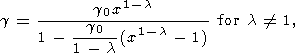 |
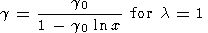 | (120) |
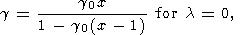 |
 where
g0 = g0(S)
and
V0 = V0(S). These
dependences of
g(x) for various
l are
illustrated in Figure 10. One can see that they are quite
sensitive to variations of
l in the interval from 0 to 1.
where
g0 = g0(S)
and
V0 = V0(S). These
dependences of
g(x) for various
l are
illustrated in Figure 10. One can see that they are quite
sensitive to variations of
l in the interval from 0 to 1.
In geophysics, the conditions close to adiabatic are realized in the convecting mantle and core, as well as in seismic wave propagation. Furthermore, the state at the initial part of Hugoniot are close to adiabatic. Adiabats of a given material form a one-parametric family of curves. In this case, the temperature and pressure are related by the adiabatic gradient tS, which, considering its definition by (10) and relations in section 2, can be written in the form
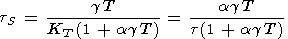 |
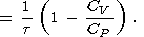 | (121) |
Typical values of tS found by (121) are given in Table 2 and Pankov et al. [1997].
Direct measurements of tS at high pressures and temperatures were made in a series of works [Dzhavadov, 1986; Boehler and Ranakrishnan, 1980; Boehler, 1982, 1983]. Chopelas and Boehler [1992] reported corrections to the Boehler [1982] initial results on tS.
We consider the basic identities and approximaions for the derivatives. Denoting by n the logarithmic volume derivative of tS and using q by (106), we have
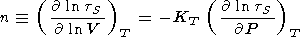 |
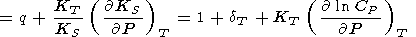 | (122) |
(compare with (45)).
Formula (122) can be represented in various forms, using q by (113), (115), and (58). It is clear that n decreases by isothermal or adiabatic compression. The simplest estimate of n is given by assuming that
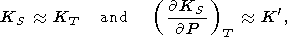 |
hence,
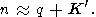 | (123) |
The typical values of
q = 1-2 and
K = 4-5 yield
n
= 4-5 yield
n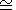 5-7.
If we neglect the last term in (122) at
T > Q, then
n
5-7.
If we neglect the last term in (122) at
T > Q, then
n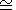 1 + dT
[Chopelas and Boehler, 1992].
1 + dT
[Chopelas and Boehler, 1992].
Changing from variables (V, S) to (V, T), the adiabatic derivative with respect to volume takes the form
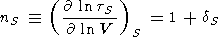 |
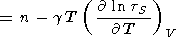 | (124) |
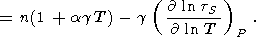 |
Approximation (89) and
n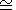 1 + dT
give
nS
1 + dT
give
nS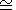 n - g.
Writing the derivative of
tS with respect to
T in the form of (29),
n - g.
Writing the derivative of
tS with respect to
T in the form of (29),
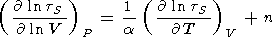 | (125) |
or after substituting
( lntS/
lntS/ T)V by (124),
T)V by (124),
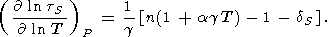 | (126) |
The values of
(( lntS)(
lntS)( ln V))P and
n (an extrinsic anharmonic contribution)
calculated by (126) and (122) are given in Table 2 and
Pankov et al. [1997].
They show that the intrinsic anharmonic term
dominates in (125).
ln V))P and
n (an extrinsic anharmonic contribution)
calculated by (126) and (122) are given in Table 2 and
Pankov et al. [1997].
They show that the intrinsic anharmonic term
dominates in (125).
Note that the tS parameter occurs in any expression when changing variables P, S to P, T : for example,
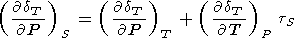 | (127) |
which was used in deriving (98).
For a moderate compression, the volume dependence of tS can be described by the power law
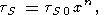 | (128) |
where n = n (T) or constant, tS0 = tS0 (T) and V0 = V0 (T). This formula was used to fit the measured tS values to P = 50 kbar and T = 1000 K [Boehler and Ramacrishnan, 1980; Boehler, 1982].
However, Chopelas and Boehler [1992], accounting for the variation of dT with V (see (46) and (47)), found that the linear dependence of lntS on V (n = mx) better describes teir data on tS than the power law, and consequently,
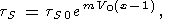 | (129) |
where constant m is determined by the approximation
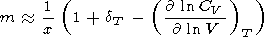 | (130) |
(see (122), where
CP is approximately
CV ). Thus, on the
condition that
( ln CV/
ln CV/ ln V)T is independent of
V, the derivative
(
ln V)T is independent of
V, the derivative
( dT/
dT/ x)T can be
found given knowledge of the
m value. Isaak [1993]
applied this
method to evaluate the derivative
x)T can be
found given knowledge of the
m value. Isaak [1993]
applied this
method to evaluate the derivative
 2KS/
2KS/ P
P T with the help of (91).
T with the help of (91).
Measured values of tS(P, T) allow us first to find the isobaric specific heat [Dzhavadov, 1986]
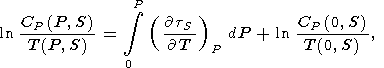 | (131) |
which is deduced from the identity
 2 T/
2 T/ P
P S =
S =
 2 T/
2 T/ S
S P.
The integral in (131)
is taken over an adiabat, and the specific heat versus
temperature,
CP(T), for
P = 0, is assumed to be known. Then,
given a reference isotherm
V(P, T0 ), from (14), we can find
the thermal EOS in the form
P.
The integral in (131)
is taken over an adiabat, and the specific heat versus
temperature,
CP(T), for
P = 0, is assumed to be known. Then,
given a reference isotherm
V(P, T0 ), from (14), we can find
the thermal EOS in the form
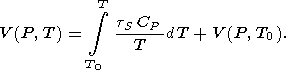 | (132) |
Conversely, given the thermal EOS and tS(T) at P = 0, (14) gives CP(T) at P = 0, and thus, the caloric EOS can be determined.